Bottles, Pressure and Temperature
DSPORT Issue #252
Text by Michael Ferrara // Photos by Joe Singleton
Feeling a little hot? Under some massive pressure? Chances are that you act differently in hot and high-pressure situations versus cooler, low-pressure circumstances. Like most chemical compounds, nitrous oxide also behaves differently based on the temperature and pressure of its environment. When the temperature of nitrous oxide is under 97 degrees Fahrenheit, it can exist as either a liquid or a vapor depending on the pressure of its surroundings. Since nitrous oxide lives in a bottle, the temperature of this container determines the pressure in the bottle. After outlining the function of the nitrous oxide bottle, we will explain the effects of temperature and pressure on a conventional nitrous-oxide system. Armed with this understanding, you will be better able to select the right bottle for your application while also understanding the potential advantages of a multi-bottle and modern push system nitrous oxide system setups.

Bottle: Storage and Pressure Setter
The function of the bottle in a nitrous oxide system is the both the obvious and the overlooked. As for obvious, the nitrous oxide bottle is the storage tank for the nitrous oxide system. A 12- or 15-pound bottle can store 20 or 50 percent more nitrous oxide than the popular 10-pound bottle. Can a system be set up with multiple bottles? Yes. In fact, multiple bottles can be setup in such a way as to deliver improved performance over single bottle systems.
What many people don’t understand is that the temperature of the bottle also sets the effective pressure that the nitrous oxide will be at once the system is activated. This is true for both single and multi-bottle conventional nitrous oxide setups. However, this is not the case for the more complex “push” nitrous oxide systems which will be covered later.
All other factors being equal, a colder bottle will have lower bottle pressure. Lower bottle pressure equates to a lower delivery pressure. Lower delivery pressure means that less nitrous will be delivered through the jet when the nitrous oxide system is activated. As bottle temperature increases (up to 97 degrees Fahrenheit), bottle pressure will also increase. This results in higher delivery pressures which increases the mass of nitrous oxide delivered for a given jet size. However, when bottle temps get too hot (above 97 degrees), the nitrous oxide in the bottle goes to a supercritical liquid state that reduces performance. Understanding how nitrous oxide lives in its two-state equilibrium is critical to extracting maximum performance and consistency from your nitrous oxide system.

Two-State Equilibrium
If the bottle temperature is under 97 degrees, the nitrous oxide in the bottle will exist in both a liquid and gaseous state. If the temperature of the storage container (bottle) is under 97 degrees Fahrenheit, one can determine the pressure of the bottle knowing the temperature or, vice versa, the temperature of the bottle by knowing its pressure. The saturated vapor pressure curve for nitrous oxide establishes the exact temperature the nitrous oxide will be at for a given pressure or the converse, the exact pressure that the nitrous will be at for a given temperature.
On a cold day where the bottle temperature drops to 40F, the pressure in the bottle will be a relatively low 520psi. The same bottle of nitrous oxide at a bottle temperature of 85F will be about 950psi.
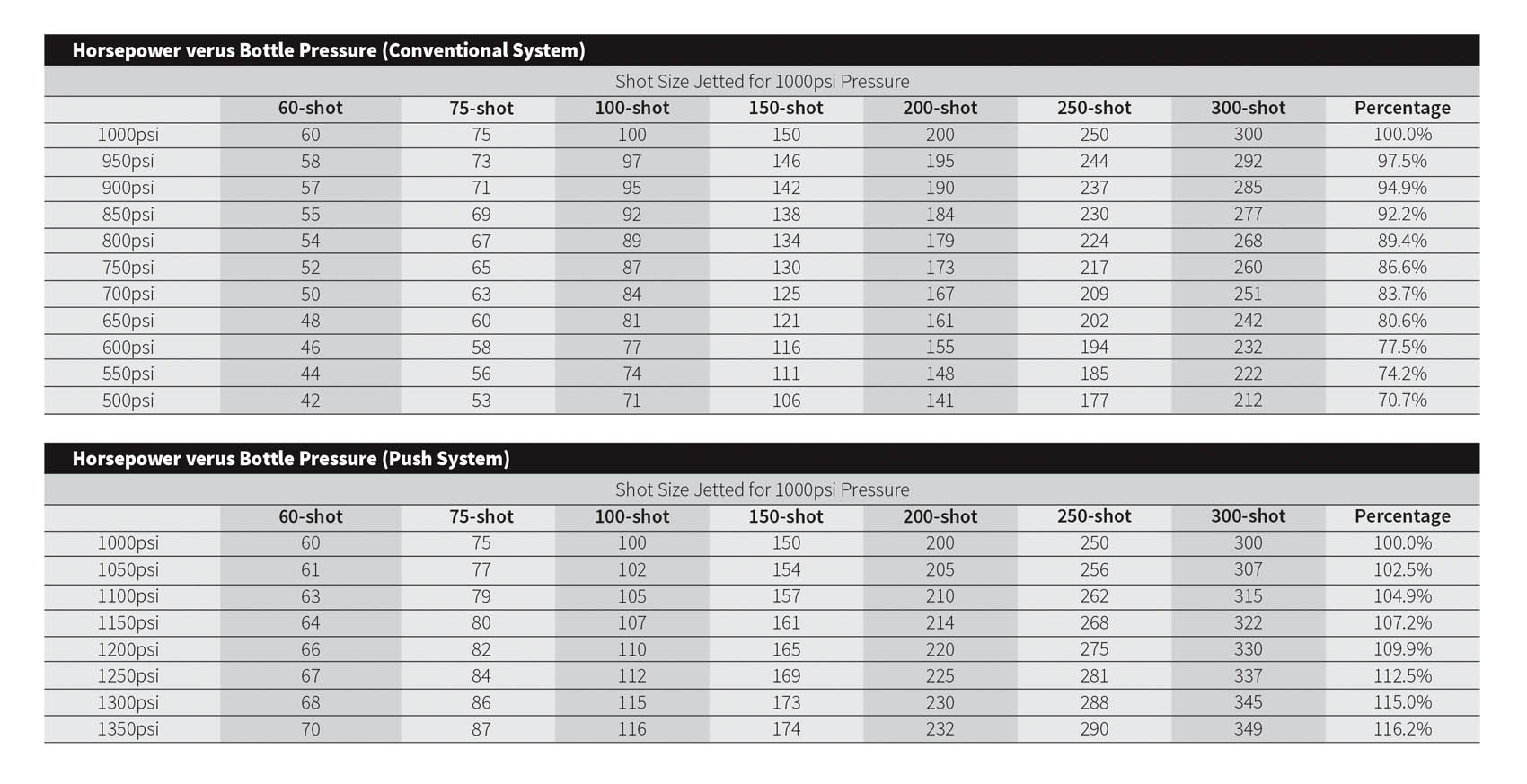
Most nitrous systems manufacturer’s supply jet size recommendations based on a specific delivery pressure. Normally this pressure is in the 950-1000psi range. When following the nitrous oxide manufacturer’s jet size recommendations, bottle pressure should be regulated (by controlling bottle temperature through a bottle heater) to that same range. A temperature switch or pressure switch can be used along with a bottle heater to heat the bottle enough to reach the target temperature or pressure and then shut off.
When the thermostat or pressure switch of the bottle heater malfunction or the outside temperatures are hot enough to bring the bottle temperature to over 97 degrees, bottle pressure will increase to over 1,050psi. When this occurs, the nitrous oxide inside the bottle no longer exists in two separate physical states (liquid and gas), instead it exists in a supercritical fluid. When nitrous oxide is in this supercritical fluid state, it will adversely affect the performance and consistency of the nitrous oxide system. As a result ,this condition should be avoided.
Cold Bottle Needs a Warm-Up
When using a conventional nitrous oxide system (non-push system), a bottle heater regulated with a pressure sensor or thermostat to keep bottle pressure in the 950- 1000psi range should be used. If the bottle temperature is too cold, pressure will be lower than ideal for the nitrous jets being used. A bottle at 40F with a pressure of 520psi will only deliver 70-percent of the mass of nitrous as a bottle at 1,040psi. That turns that “200-shot” into just a 140-horsepower shot if the additional fuel supplied when the nitrous system is activated is properly trimmed for the lower bottle pressure. When the fuel is not trimmed when bottle pressure drops to low levels, excessively rich fuel mixtures might take that “200-shot” down to only deliver an additional 100 horsepower or less. Due to the massive swing in nitrous oxide delivery based on bottle temperature, a bottle heater regulated by bottle temperature and/or bottle pressure should be considered a required element of any conventional nitrous oxide system. For push- systems, no bottle heater is required. Instead, the nitrous-oxide bottle simply needs to be setup to stay below 97 degrees. In these applications, an insulated blanket may help the bottle stay cooler than the location such as a trunk that gets warmer during the day.

Supercritical = Super-crappy
When any nitrous-oxide system is activated when the nitrous oxide bottle is above 97 degree bottle temperatures (bottle pressure over 1,050psi), the non-liquid/supercritical fluid nitrous oxide released into the engine creates a lower horsepower gain. This holds true for both conventional and push nitrous oxide systems. This is the result because nitrous oxide in the bottle exists in a supercritical fluid state that has a lower density than liquid or gaseous nitrous oxide. If you are familiar with how turbochargers become ineficient at boost pressures beyond their designed operating range, the same happens here when bottle temps above 97 degrees boost a bottle to pressures beyond 1,050psi. While the boost pressure (bottle pressure) is higher, the mass flow of pounds of nitrous oxide delivered to the engine is lower due to the drop in density of the supercritical fluid state.
Dropping that Pressure
Once a nitrous oxide system is activated and nitrous oxide is leaving the bottle through the bottle valve, the bottle will cool and the bottle temperature will drop. You may have noticed this same phenomenon happening when you spray a can of compresed air used to clean your electronics. You spray the compressed air and what starts out as a room temperature can that puts out a strong spray turns into a cold can that puts out a fraction of the pressure. Again, the nitrous oxide temperature and pressure relationship will follow the saturated vapor pressure curve.
Another way to drop the pressure in the bottle is to purge the nitrous system. Activating the purge solenoid in the system creates the same cooling effect on the bottle, but the nitrous oxide is released into the atmosphere instead of the engine. Purging also helps to remove latent heat in the lines and solenoid. This provides the benefit of allowing the nitrous oxide to extract more heat from the intake charge when the system is activated.

Bigger Bottle or Multi-Bottles
So besides allowing more seconds of being able to “squeeze,” bigger bottles will also maintain bottle pressure and temperature more consistently during the activation period of the system. Depending on the size of the horsepower “shot” and the length of the squeeze time, the bottle will cool a certain amount meaning the pressure and temperature of the bottle will drop during the run. If the starting pressure is 1,000psi and a 150-shot is jetted in the system, the bottle pressure in a 10-pound bottle might drop near 800psi a er 8-to-10 seconds of being on the squeeze. With a larger 15-pound bottle, the drop in pressure might only go down to 850-850psi while a pair of 10-pound bottles might keep the bottle pressure closer to 900 psi at the end of the same run. We’ve included a chart that shows the difference in power delivery from the nitrous oxide as bottle pressure drops.
What does a nitrous oxide bottle cost? Your basic 10-pound bottle when purchased separately will run about $250. Upgrading to a 15-pound bottle will only cost about $30 more. This is a very cheap upgrade that will improve the performance of your nitrous oxide system while also increasing the amount of nitrous oxide you have on tap by 50%. It’s about the best bang-for-the-buck upgrade you can make to a nitrous oxide system. If you can fit it, do it! Using a carbon-fiber wrapped bottle will set capacity to 12 pounds (a 20% gain in capacity over standard 10-pound bottle), but it will generally be about a $300 premium over the standard 10-pound bottle. The carbon-fiber bottle will save 7.5-pounds of weight compared empty to empty or 5.5 pounds of savings full to full.
When choosing your bottle, be sure to select the best bottle valves for your particular application. The 90-degree turn type Outlaw valves are usually a $200 premium over standard valves. While you may not need the additional flow potential of an Outlaw valve if you are using anything under a 250-shot, this valve is my preferred selection because it’s easy to see if the valve is on or o with just a quick glance. Having been at over 120 IDRC races over the past 25 years, there have been way too many races lost because someone thought the bottle was open when the twist valve was actually closed. Personally, I’d rather pay the extra $200 for an Outlaw type valve and then step the outlet down to -4AN and run a -4AN line even though there won’t be a performance increase at nitrous oxide levels below a 250 horsepower shot.
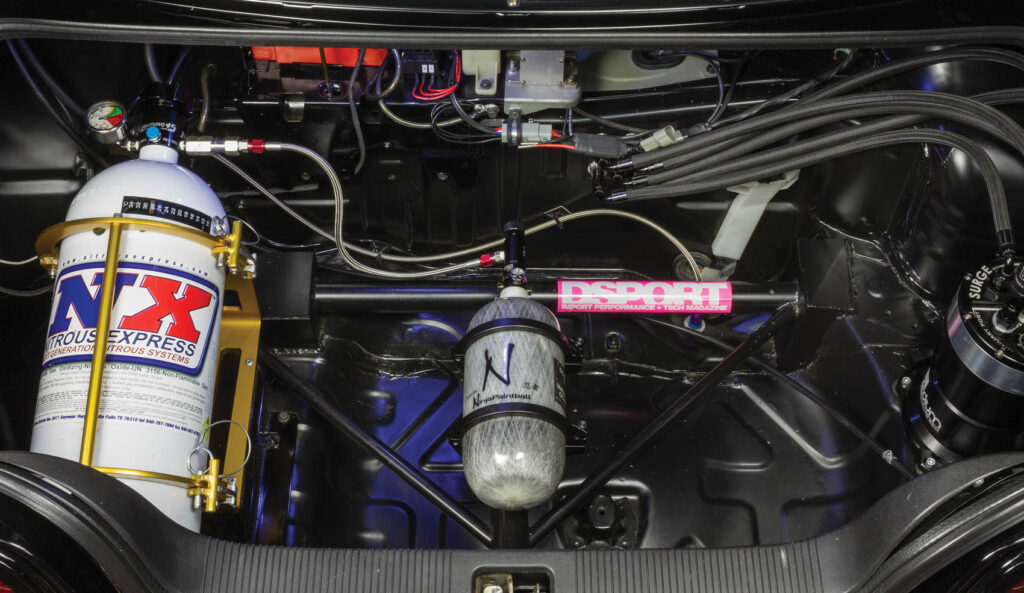
Let’s Push It
Where conventional nitrous oxide systems rely on the temperature of the nitrous bottle to determine the delivery pressure of the nitrous oxide in the system, a “push” nitrous oxide system uses pressurized regulated air or nitrogen gas in a separate pressurized bottle (usually pressurized to 3,000 or 4,500psi before being regulated) to set the delivery pressure (usually between 950psi and 1,250psi). While building a push deliver system is more complex, it does have its advantages. These advantages include: the ability to increase delivery pressure of nitrous oxide above 1,050psi; the possibility to deliver a higher mass of nitrous oxide through a given jet or solenoid by running these extended delivery pressures; allows a higher percentage of the nitrous oxide in the bottle to be used before performance drops o ; more consistent delivery pressure on nitrous system and there is no need for a bottle heater in the system. In fact, the nitrous oxide bottle in a push system needs to be kept cool and never allowed to be above 97 degrees.
The drawbacks to push systems are that it adds cost and complexity. A push system will require a compatible bottle valve on the nitrous bottle, a 3,000psi or 4,500psi rated bottle for the air or nitrogen gas used in the system, a regulator attached to the push bottle that regulates the pressure down to the 950-1,350psi range and the associated high pressure plumbing. A check valve may also be part of the system to ensure that nitrous oxide doesn’t flow out of the bottle if there is a leak in the push system. The cost of the push system can be as much or more than the cost of a basic nitrous oxide system. You will also need to find a location that can fill the push bottles with either air or nitrogen to the rated pressure of 3,000 or 4,500psi depending on the bottle rating. If you are involved in paintball or scuba diving, you may already have your refill location. Ultimately, a custom designed push system requires some experience. We’ve used our custom designed push system on our R33 GT-R for several years without any issues. If you don’t know if you will go this route now or in the future, a good idea is to future proof your system by purchasing a bottle valve that has both a gauge and a “push” port designed into the valve from the start.

The Bottom Line
It’s just a phase. This could be referring to your insistence on dressing up while you
play your favorite video games or it could also apply to what’s going on with the nitrous oxide in its bottle. For conventional nitrous oxide systems, keeping the bottle within the optimum temperature range will establish the ideal pressures to delivery the nitrous oxide into your engine for maximum performance. This is where the Goldilocks porridge logic applies. The bottle temperature cannot be too hot (about 97 degrees) or too low (under 85 degrees) if maximum repeatable performance is the goal.
Nitrous FAQ
1. Q: Does my bottle pressure indicate the level of nitrous oxide in the bottle?
A: No. The only way you can determine the amount of nitrous oxide in the bottle is by weighing the bottle.
2. Q: Is it OK to overfill my bottle? Can I put 11 pounds in my 10-pound bottle?
A: No. This is not a good idea at all. While a compressed gas bottle is usually only filled to between 65 to 75 percent of its total capacity, this additional space is needed for safety and for the saturated vapor curve to be properly followed.
3. Q: Can I overheat my bottle?
A: Of course. A bottle can get overheated (Above 97F) by just being placed in a location that warms the bottle up above this threshold. The heater circuit used with a bottle heater can also have an issue that keeps the heater on beyond the temperature desired. If either the pressure switch or thermostat fails, the heater can remain on an get the bottle too hot. Ultimately, a bottle that gets too hot may trigger the safety valve.
4. Q: Is medical grade nitrous oxide better than Nitrous+ or similar motorsports mixtures?
A: Not going to make a difference in performance. Just use the Nitrous+ or similar mixture to keep substance abusers away from your nitrous oxide.
5. Q: Can nitrous oxide be used with E85 or other ethanol blended fuels? Can nitrous oxide be used on engine running methanol fuel?
A: Yes and Yes. The amount of nitrous oxide added to the engine will remain the same for a given horsepower “shot” of nitrous, but the amount of fuel added will be more than with gasoline. On a wet system, this will mean that the fuel jet size for E85 will be a larger number than the fuel jet size for gasoline. The fuel jet size for methanol will be even bigger than the fuel jet size for E85 or gasoline.
6. Q: Is it OK to use a torch to heat up the bottle?
A: No. Never use a torch to heat a bottle. The best way to heat a bottle outside of the vehicle is to place it in a warm water bath with the water temperature at the desired bottle temp. When a bottle is in the vehicle and needs to be heated, use a bottle warmer with a pressure switch installed in the circuit. Be sure that the bottle warmer is placed on the bottom of the bottle where liquid nitrous oxide stays and not the top portion of the bottle.
7. Q: I have two nitrous oxide bottles that are half full. Is there an easy way to fill the one bottle and from the other?
A. It’s best to just get both bottles topped o at your local nitrous oxide dealer. However, if a situation arises where you must get the nitrous out of one bottle and into another, there is a way. The process will require an accurate scale and a -4AN to -4AN line. For the bottle that has space to receive additional nitrous oxide, the bottle can be cooled in a ice bath Let the bottle cool until the pressure drops to under 600psi. For the bottle that will be filling the cool bottle, it can warmed up until the pressure it as high as 1,000psi (900psi is usually good enough). The cold bottle and warm bottom should then be connected by the -4AN to -4AN line. Make sure the warm bottles is positioned in a standing position.
One the lines are tightened; the receiving bottle can have the valve opened completely. Then the warm donating bottle can have its valve opened to begin the fill of the cold bottle. It’s best if the cold bottle can be on the scale to verify that its gaining weight and to make sure it’s not getting overfilled. Again, it’s much safer to just get both bottles topped off.



